
Published: May 29, 2019 | By Sydney Valentino
In the following MIRA Highlight of the Month, Sydney Valentino interviews Mitra Shokrollahi, who is in the second year of her program Master’s with the supervision of Dr. Jan Huizinga, and defending soon in June!
Q: Tell me about the research project you are currently working on.
A: I have been working on gut serotonin signalling involved in colon healthy function using the rabbit animal model. Serotonin is usually known as a chemical produced by the nerve cells. However, more than 90% of the body’s serotonin is produced in the wall of the digestive tract. Serotonin plays an important role in the regulation of motility and secretory function of the intestine and is produced by a group of specialized cells in the wall of the colon. The focus of my research has been on pharmacological stimulation of a sub-class of serotonin receptors located in the wall of the colon with the aim of regulating colonic function from within. This could help in improved management of disorders such as Irritable Bowel Syndrome (IBS) and chronic constipation. This research is translational and we also use High-Resolution Colonic Manometry (HRCM) in humans to test if the results achieved in our animal studies are seen in humans as well.
Q: How many people actually take this drug and what is the prevalence of poor side effects?
A: First let’s start by explaining, this “class” of drugs called serotonin type 4 (HT-4) receptor agonists interact with serotonin type 4 receptors in the intestine, to increase intestinal movement, and further up the line, to increase stomach emptying. There is a number of these drugs with various effects and potency. They are prescribed to individuals with hypomotility disorders including but not limited to chronic constipation and irritable bowel syndrome constipation dominant (IBS-C). The prevalence of chronic constipation has been reported to be between 2-27% of the populations and IBS affects more than 11% of the global population. Therefore, a great number of people are affected. A previous study has reported the number of serious adverse events associated with different prokinetic drugs some of which are currently still prescribed by many doctors. Based on this report, Metoclopramide, for instance, has caused 17356 adverse events, of which 6.5% resulted in death as of 2018.

This shows Mitra’s hard work, a whole isolated proximal rabbit colon in the organ bath ready for testing.​​
Q: Can you tell us about your research and how it relates to the aging population?
A: As we age, not only our skeletal muscles gradually lose function but also our smooth muscles which are present in the walls of hollow organs like the urinary bladder, arteries, veins, stomach and intestines. This gradual loss of function of the smooth muscles reduces the healthy movements of the large intestine and can result in various colonic disorders such as chronic constipation. In severe cases, colostomy could be recommended which is a surgical procedure to create an opening from the colon to the outside of the body to divert waste from the digestive system. It is not a pleasant experience as you would need to live with a colostomy bag.
In less severe cases, common medications currently prescribed are oral prokinetic drugs that are effective in managing the symptoms. However, despite their effectiveness, these drugs have long been associated with adverse cardiovascular and renal side effects, cardiac arrest and death. Some oral prokinetics have already been removed from the market because of these unexpected side-effects.
The elderly are a high-risk group who manifest the highest incidents of age-related gastrointestinal disorders while at the same time having cardio-vascular and renal conditions. Moreover, the metabolism of many drugs by the liver is also impaired in the elderly. Therefore, the use of current oral prokinetics for the aging population is riskier than other age groups.
Most oral drugs are absorbed in the upper gastrointestinal tract, go through metabolism in the liver (hepatic drug metabolism) and are “systemically” absorbed via the circulatory system. So, the entire body is affected not specifically only the organ we intend to treat. This is problematic because the drug circulates throughout the body, reaching all other organs where it is not needed and hence, it may cause unwanted effects. Results from this study support the notion of using the gut wall serotonin signalling to regulate the function of the colon instead of the systemic drug administration.
Q: How can this research be applied to have a real-world impact?
A: The most important clinical implication of this research is that instead of the conventional methods currently used for administrating such prokinetic drugs, we can develop nano-particles or other systems to deliver them directly to the patient’s colon without the drug is first absorbed in the stomach.
This will significantly minimize the risks of adverse side-effects associated with these drugs which are very meaningful especially for the aging population. Besides, previous studies have shown that this method is not only safer but could also be more effective.
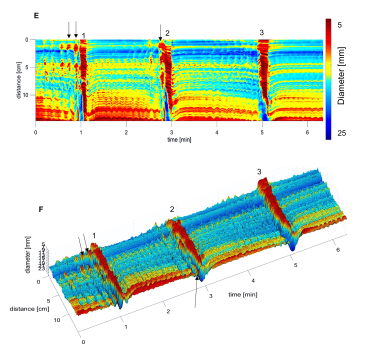
This figure is a 3D map of the activity of the rabbit colon after intraluminal drug administration. It shows typical propulsive motor patterns called the colonic motor complex which was evoked by the luminal drug.
Q: What is the ultimate goal of this research?
A: The ultimate goal of this research was to show that activation of a type of serotonin receptors in the gut-wall from within does, in fact, enhance the colonic function. We have recently published the results of our animal model studies (https://onlinelibrary.wiley.com/doi/10.1111/nmo.13598) where we show this to be true and our human studies are in progress. Therefore, it is possible to use this signalling pathway to our advantage and develop colon-specific drug delivery systems.
Thank you to Mitra, for helping me highlight this awesome research, that dives deep into the intestinal system to understand the ways new medicinal treatment, typically for our aging populations, may be administered. In September 2019, she will be the University of Toronto for her PhD studying the impact of genome spatial organization and the environment on genome expression and stability.
If you have an interest in Mitra’s current work,or future work involving the genome, feel free to contact her at shokorlm@mcmaster.ca
This article was first published by the MIRA Trainee Network. Read the ​original article.​​

