Published: August 18, 2021 By: Jessie Park
An interdisciplinary team of researchers from McMaster and SickKids are developing a cutting-edge lung model that can better respond to viruses and drug treatments, giving scientists a tool to advance research in lung conditions like COVID-19, cystic fibrosis and allergens for asthma and air pollution.
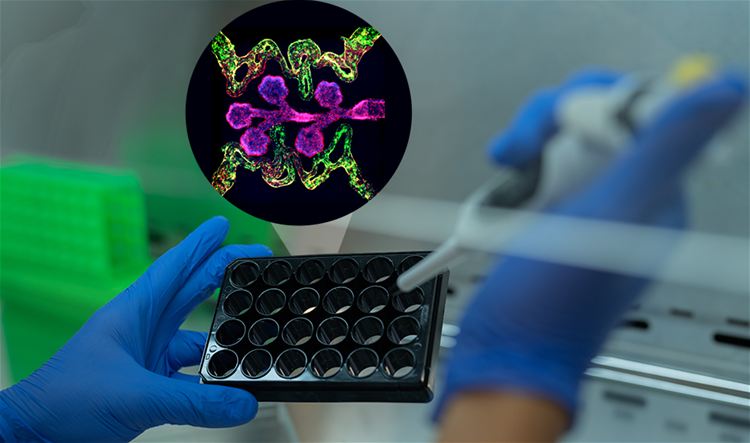
The new bioengineered lung model – coined a “clinical trial on a plate” by the research team – will replicate key features of the human lung, including specialized cells, surrounding blood vessels and life-like immune functions.
This project recently received $1M in funding as the winner of the National Sanitarium Association research grant, which supports important new areas of research in pulmonary science and medicine. A preprint version of the research paper was published this week in bioRxiv.
“To better prepare for future pandemics, more sophisticated human lung models are needed to study disease and treatments with more precision. Our lung model platform will improve clinical trial designs for COVID-19 and beyond, with broad applications to drug development, immunology and developmental biology of the lung,” says Boyang Zhang, an assistant professor in Chemical Engineering who is leading this work at McMaster in collaboration with leading experts Jeremy Hirota and Karen Mossman from McMaster, and Amy Wong from SickKids and the University of Toronto.

Even before COVID-19, the researchers had started combining their expertise to advance lung models – stem cell-derived organoid technology from Wong’s lab, virology expertise from Mossman’s lab, lung biology and immunology expertise from Hirota’s lab, and organ-on-a-chip engineering from Zhang’s lab.
“With COVID, we certainly felt the urgency to integrate these different technologies that are already out there to create something on the next level,” says Zhang, adding the team is working towards bringing their work to a Biosafety Level 3 lab, which is needed for COVID-19 research.
HOW DOES IT WORK?
The “clinical trial on a plate” lung model will combine the best features from two innovative bioengineered organ systems: stem cell-derived organoids and organ-on-a-chip models.
At SickKids, Wong’s lab developed a way to have artificial stem cells “turn into” the different cells of the lungs, mimicking key developmental steps of the complex organ. The high variety of cell types is critical to accurately model an organ in 3D, according to Wong.
“With the incredible team at McMaster, we will integrate our stem cell-derived lung organoid model with immune cells and a vasculature to create a more advanced preclinical lung model for disease modeling, and to better predict treatment outcomes for each individual,” says Wong, an assistant professor in laboratory medicine and pathobiology at the University of Toronto. She conducts stem cell biology and cystic fibrosis research at SickKids.
The organ-on-a-chip system developed in Zhang’s lab gives scientists more control over the micro-environment where the cells are cultured. For example, the cells can be stretched and immersed in fluid to test responses to drug treatment. The downside is these models have over-simplified cell populations which don’t translate as well in 3D.
“To overcome the trade-offs between the two techniques, we’re combining them to develop a more advanced system,” says Zhang.

The cluster of cells in the “clinical trial on a plate” model is created on a plastic plate with 384 wells, or pockets. Each model is no bigger than a grain of rice.
“It takes the same amount of effort to make 100 tissues as it does to make one tissue,” says Zhang. “For drug testing purposes, we don’t need the tissue to be very big and we purposely keep them small so we can use less cells to create the model. You’re saving on material cost, but you get the same information out of it.”
RESPONDING TO VIRUSES
Another breakthrough of the new lung model is its ability to replicate air and blood circulation, virus infections and immune responses.
The team developed a network of blood vessels around the model, which is key to studying immunity in the lung since immune cells live and travel in the blood, explains Hirota, an assistant professor in respirology and Tier 2 Canada Research Chair in Respiratory Mucosal Immunology at McMaster.
“Immune responses during infection are dynamic and involve multiple cell types, yet historically, the cell models we have used to study infections were static and restricted to one cell type,” he adds, noting the team’s proposed model will use multiple immune cell types.
“This is an opportunity to revolutionize how we study and understand immune responses to infectious agents, and the development of drugs for these infections.”
With the new funding from the National Sanitarium Association, the team will spend the next five years developing and testing the lung model for clinical use.
Earlier work on this project was funded by the Canadian Institutes of Health Research (CIHR), with an additional grant from the Canadian Foundation for Innovation (CFI) supporting the purchase of key equipment to advance the research – a high-content confocal microscope for high-throughput imaging, and a robotic liquid handling system to automate the production process.
“McMaster is recognized as a national and global leader in respiratory research and we’re so pleased that the National Sanitarium Association recognized the game-changing potential of our work,” says Karen Mossman, McMaster’s vice-president, research, and professor of medicine.
“Our ability to work across disciplines and institutions – the kind of collaboration that is synonymous with McMaster – significantly increases our ability to advance the health and well-being of citizens the world over.”
This research supports McMaster’s Global Nexus for Pandemics and Biological Threats, an international network of scientists, clinical health and medical specialists, engineers, social scientists and other experts working collaboratively to prevent future pandemics and mitigate global health threats.
This article was first published on Brighter World. Read the original article.

